
Cavity Ring-Down Spec.
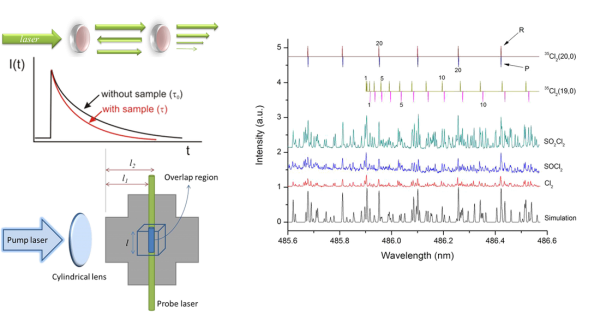
As an emerging absorption technique, Cavity Ring-Down absorption Spectroscopy (CRDS) is based upon the measurement of the decay rate of light trapped in an optical cavity with high reflectance. When a pulsed laser radiation is guided into an optical cavity, the small amount of light trapped inside the cavity reflects between two highly reflective mirrors (R>99.9%) with a small fraction transmitting through each mirror for each pass. The decay rate of the light leaking out of the cavity is related to the absorption coefficient of the sample in the cavity. Therefore, the CRDS method may ignore fluctuation of incident radiation intensity, with better sensitivity than conventional absorption methods due to a longer optical path.
Atmospheric halogen chemistry has drawn much attention, for the halogen atom (X) playing a catalytic role may cause severe stratospheric ozone depletion. atomic X elimination from X-containing hydrocarbons is recognized as the major primary dissociation process upon UV-light irradiation, whereas a direct elimination of X2 product has been seldom discussed or remained as a controversial issue. For the past years, we have detected X2 primary products using CRDS in the photolysis at 248 nm of a variety of X-containing compounds, focusing on bromomethanes (CH2Br2, CF2Br2, CHBr2Cl, and CHBr3), dibromoethanes (1,1-C2H4Br2 and 1,2-C2H4Br2) and dibromoethylenes (1,1-C2H2Br2 and 1,2-C2H2Br2), acyl bromides (BrCOCOBr and CH2BrCOBr), diiodomethane (CH2I2), thionyl chloride (SOCl2), and sulfuryl chloride (SO2Cl2). The optical spectra, quantum yields, and vibrational population distributions of the X2 fragments have been well characterized. With the aid of ab initio calculations of potential energies and rate constants, the detailed photodissociation mechanisms may be comprehended. Such studies are fundamentally important to gain insight into the dissociation dynamics and may also practically help to assess the halogen-related environmental variation. A review will soon appear in Phys. Chem. Chem. Phys. (Perspective, 2014)
