Research Topics
Our research concern on gaseous phase photodynamics. We also study quantum dot properties and single molecule detection.
-
Set up scientific apparatus
-
Laser optics
-
Vacuum technology
-
Data acquisition and manipulation
Now we collaborate with Prof. Vincenzo AquiIlanti 's group in Italy and Prof. Oleg Vasyutinskii in Russia. You may have chance to expand you view.
Gas Phase Photolysis Dynamics
Ion-Imaging
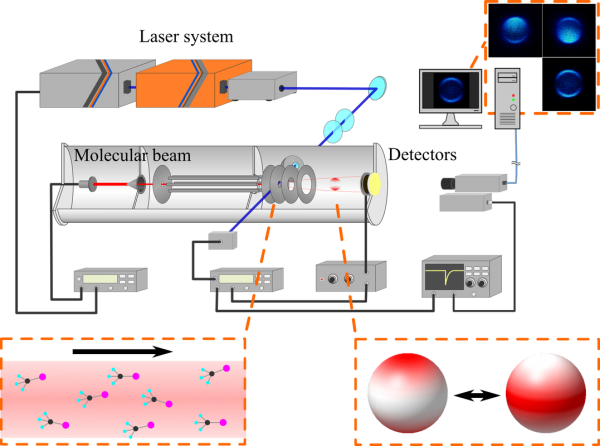
We are studying on photodissociation of molecules, how it is taken place and what undergoes in a reaction. The photodissociation is, generally, one of the simplest chemical reactions; nevertheless it gives a lot of information on molecular structure, chemical bondings, energy distributions and so on. Even more noteworthy thing is that photodissociation is ubiquitous, there is a plenty of sunlight in every day time. Photochemistry takes enormous effects on our environment although it is invisible. To get understand about further complex chemical reactions, and also to know about our environment, it is necessary to study on photochemistry.
Currently we combines ion imaging technique with molecular orientation to investigate the role of molecular geometries in photodissociation. We are expecting that the molecular orientation technique would unveil hidden facts under the thermal randomizations.
Time-Resolved FT-IR
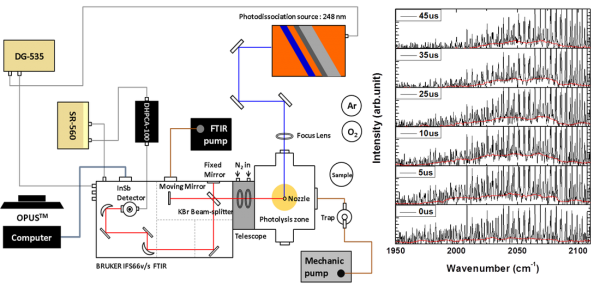
Other than the ester family, aliphatic aldehyde is a second molecular series that we are looking into this newly found pathway. Aliphatic aldehydes are known to be present in the atmosphere through automobile exhaust or photooxidation of organic compounds. Investigating their photodissociation mechanisms has been a main theme in photochemistry. Among these aldehydes, formaldehyde and acetaldehyde were the only two that have been found to carry roaming signature in the photodissociation. Like the tight transition state (TS) mechanism, there exists a first-order saddle point for the roaming process, showing one small imaginary (harmonic) frequency along with some small frequency modes. Propionaldehyde , as a member of aliphatic aldehydes, shows similar chemical properties to its smaller counterparts, but its photochemistry is much less investigated.
In the past years, we employed time-resolved Fourier-transform infrared (FTIR) emission spectroscopy to probe the HCO and CO fragments in propionaldehyde at 248 nm. Finally, the theoretical methods are performed in conjunction with the experimental findings to clarify dynamical complexity in photodissociation of propionaldehyde. We have found that the roaming pathway dominates the molecular products of CO + C2H6. This work implies that roaming mechanism plays an increasingly important role in aliphatic aldehydes, as the molecular size becomes larger. The results are published in J. Phys. Chem. Lett. 5, 190 (2014) and J. Chem. Phys. 140, 064313 (2014)
Cavity Ring-Down Spec.
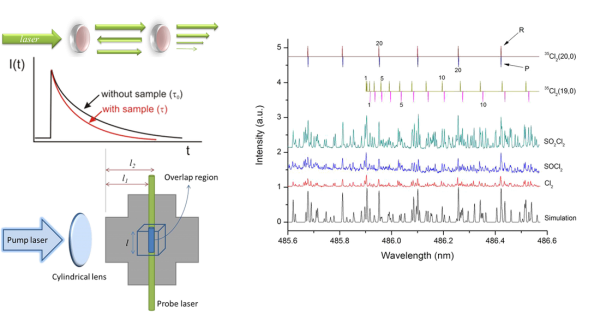
As an emerging absorption technique, Cavity Ring-Down absorption Spectroscopy (CRDS) is based upon the measurement of the decay rate of light trapped in an optical cavity with high reflectance. When a pulsed laser radiation is guided into an optical cavity, the small amount of light trapped inside the cavity reflects between two highly reflective mirrors (R>99.9%) with a small fraction transmitting through each mirror for each pass. The decay rate of the light leaking out of the cavity is related to the absorption coefficient of the sample in the cavity. Therefore, the CRDS method may ignore fluctuation of incident radiation intensity, with better sensitivity than conventional absorption methods due to a longer optical path.
Atmospheric halogen chemistry has drawn much attention, for the halogen atom (X) playing a catalytic role may cause severe stratospheric ozone depletion. atomic X elimination from X-containing hydrocarbons is recognized as the major primary dissociation process upon UV-light irradiation, whereas a direct elimination of X2 product has been seldom discussed or remained as a controversial issue. For the past years, we have detected X2 primary products using CRDS in the photolysis at 248 nm of a variety of X-containing compounds, focusing on bromomethanes (CH2Br2, CF2Br2, CHBr2Cl, and CHBr3), dibromoethanes (1,1-C2H4Br2 and 1,2-C2H4Br2) and dibromoethylenes (1,1-C2H2Br2 and 1,2-C2H2Br2), acyl bromides (BrCOCOBr and CH2BrCOBr), diiodomethane (CH2I2), thionyl chloride (SOCl2), and sulfuryl chloride (SO2Cl2). The optical spectra, quantum yields, and vibrational population distributions of the X2 fragments have been well characterized. With the aid of ab initio calculations of potential energies and rate constants, the detailed photodissociation mechanisms may be comprehended. Such studies are fundamentally important to gain insight into the dissociation dynamics and may also practically help to assess the halogen-related environmental variation. A review will soon appear in Phys. Chem. Chem. Phys. (Perspective, 2014)
Single-Molecule
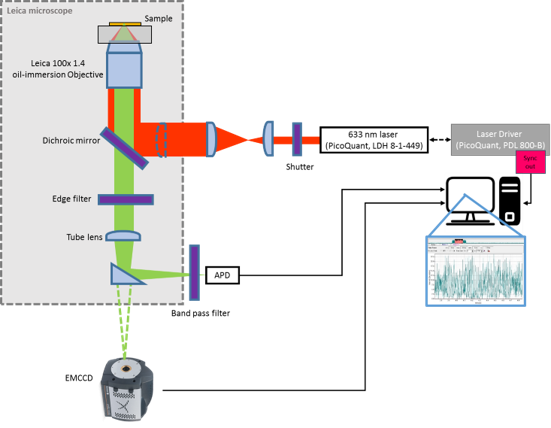
We use fluorescence single molecule to serve as a prober, which can be measured with high spatial resolution in non-invasive way. With blinking behavior, we can obtain fluorescence lifetime. Thus, probed lipid dynamics and oxygen gradient spread over single cell can be investigated.
Lipid Dynamics
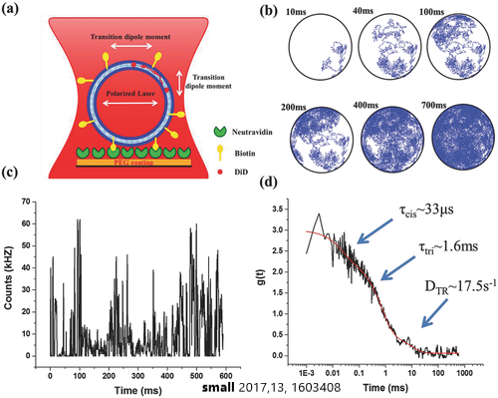
Micro-Fluidic Channel
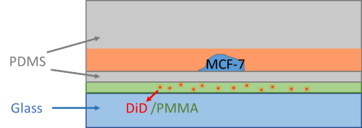
Design of a Microfluidic Channel in Application of Oxygen Spread over Single Cancer Cells
Use O2-sensitive dye (DiD) to sense the environmental oxygen concentration over single cancer cell
Material
Chemical-/Bio- Sensors
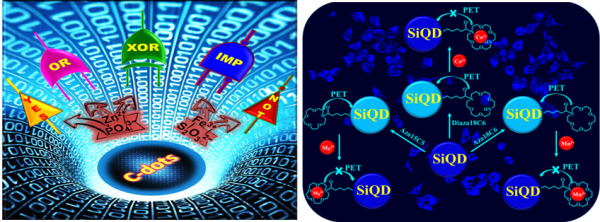
Recently, Carbon-dots (C-dots) and Silicon quantum dots (SiQD) received extensive attention due to its fascinating significance for the development of low-cost and high sensitivity chemical and biosensors systems. C-dots and SiQD exhibits tremendous characteristics such as non-toxicity, biocompatibility, photostability, facile, rapid and low cost for synthesis, easy for surface modification, and excellent photoluminescence properties. Due to these unique properties, developing C-dots and SiQD as a new material to perform chemical and biosensors should be worthwhile. We are developing a facile detection method for metal ions and anions based on the mechanism of fluorescence resonance energy transfer (FRET) and electron transfer processes.
we have demonstrated that the C-dots based fluorescent off - on (Fe3+ - S2O32−) and on - off (Zn2+ - PO43−) sensors for the detection of metal ions and anions. The sensor system exhibits excellent selectivity and sensitivity towards the detection of biologically important Fe3+, Zn2+ metal ions and S2O32−, PO43− anions.
Catalysts
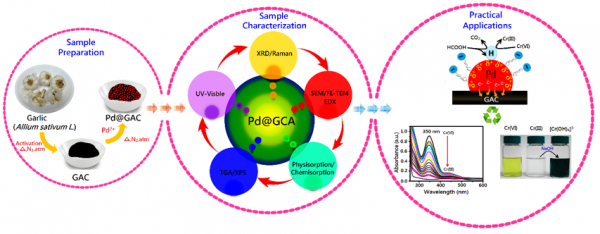
Noble metals supported on porous carbon atoms have attracted considerable attention owing to their potential applications as catalysts.The reduced particle size greatly enhances the surface-to-volume ratio, and facilitates the catalytic performance of the noble metals. However, the smallsized metal nanoparticles (MNPs) bring about some inevitable problems in separation and recycling. To overcome this difficulty, many efforts have been devoted to immobilize MNPs onto the surface of various solid supports.In past decades, palladium nanoparticles (Pd NPs) supported on porous carbon spheres (PCS) have shown fascinating catalytic activity for various organic reactions, electrochemical, and dye-sensitized solar cells (DSSC) applications.
Due to their unique properties, Pd-based carbon nanomaterials exhibit high thermal stability, good electrochemical activity, solvent resistance, large surface area, low pollution and ease of recycling, thereby attracting a wide range of applications in materials sciences and chemistry.Recently, carbon-supported Pd NPs have been demonstrated to be a viable way of removing dyes and other organic pollutants from the environment.