
Vibrational Spectra Calculations
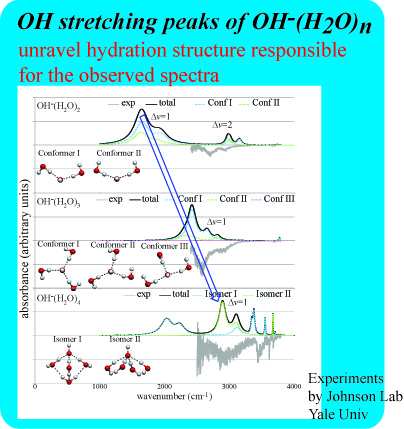
The strong ionic hydrogen bond between OH- and local water molecules causes distortions in the hydrogen bonding network compared to pure water, and the exact number of waters in the first solvation shell of aqueous phase hydroxide has been controversial. Utilizing the newly developed multidimensional local mode model with our width calculations we simulated the vibrational spectra of OH-(H2O)n n=2-4. Although the exact peak positions are not perfect, our calculation reproduces the general shape of the experimental spectra
